SUSTAINABILITY
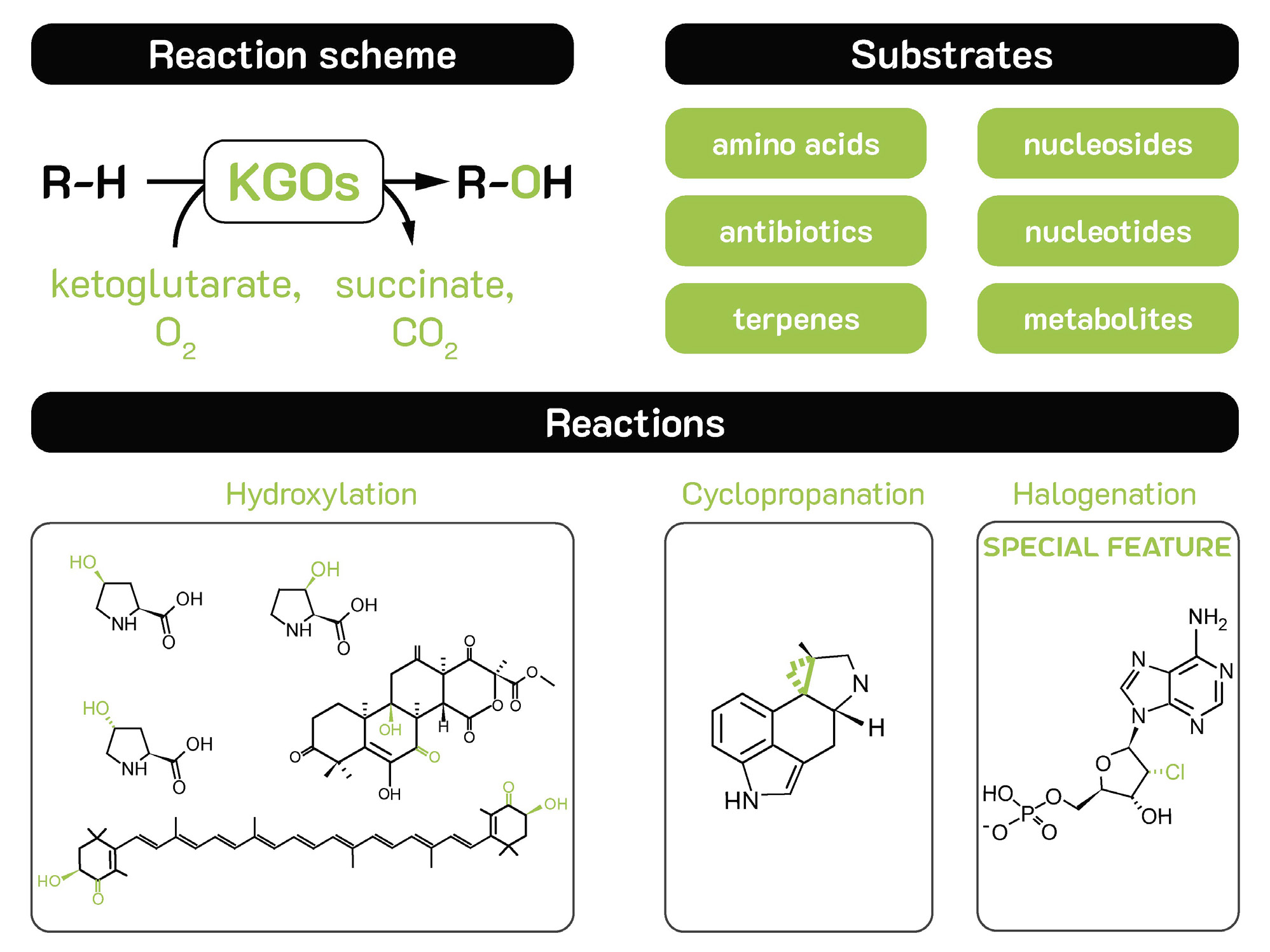 Figure 2 - Reaction scheme, substrates & reactions of KGOs .
Figure 2 - Reaction scheme, substrates & reactions of KGOs .
available in kg quantities and capable of catalysing oxygenation reactions for a broad range of compounds. The first processes used the so-called P450 monooxygenase family, which in parts are still used today – albeit not for processes beyond 100 kg scale, due to the inherent instability of the biocatalyst. Today, more promising alternatives exist and Aminoverse offers two types of biocatalysts that fulfil all criteria.
Fe(II)/α-ketoglutarate dependent oxygenases (KGOs) have been evolved by nature to act on metabolic compounds. As such, these biocatalysts have an inherent affinity for amino acids, nucleosides and DNA , antibiotics, alkaloids and terpenes (Figure 2).
As the name implies, unspecific peroxygenases (UPOs) act on a broad spectrum of substrates, which currently covers more than 400 different compounds, including steroids, terpenes and chemical building blocks like pyridines and heterocycles (Figure 1). The oxygen donor is hydrogen peroxide instead of molecular oxygen, which facilitates both handling and reagent costs significantly.
Nature evolved UPOs to withstand harsh environmental conditions. This innate stability makes them ideally suitable as biocatalysts even for water-insoluble compounds, because they can resist organic solvents typically up to 20 %v/v.
KGOs primarily catalyse siteselective C-H hydroxylation reactions, but they are also able to catalyse demethylations, ring formations, rearrangements and even halogenations using chlorine, bromine and iodine. They have already been scaled to tonne-scale biocatalytic processes.
As well as targeted hydroxylation, UPOs are also able to form ketones and even carboxylic acids, oxidise heteroatoms and demethylate. Recent scientific breakthroughs in the production of UPOs ensure kg-scale supply to facilitate the manufacturing of oxyfunctionalised products at tonne scale.
KGOs are particularly robust biocatalysts, because they do not rely on a heme-cofactor or complex domains as, for instance, in P450 monoxygenases. Unlike UPOs, however, KGOs are not suited for low-margin products, as they require ketoglutarate as a sacrificial cofactor, which needs to be considered in each business case.
UPOs & KGOs
UPO-catalysed reaction
A study by Heckmann et al affords some insights into how UPOs can
MAR /APR 2025 SPECCHEMONLINE.COM
59