David Schönauer , CEO and founder of Aminoverse , shares insights on how to integrate biocatalysis into the synthesis of oxygenated products
, the chemical industry faces a growing demand for more sustainable products. Ideally, the entire synthesis route from renewable starting materials to finished product meets new environmental standards, while minimising side-product formation and (toxic) waste streams.
Optimised over millions of years of evolution, biocatalysts show optimal performance under physiological conditions, i.e. in aqueous solutions, at ambient temperature and ambient pressure. They are biodegradable and as such offer ways to catalyse chemistry in a non-hazardous and non-toxic way.
At the same time, the fierce competition within the market and the tense economic situation toughen the challenge of meeting economic targets, especially since innovative pharmaceutical assets like PROTACs and ground-breaking antibiotics keep increasing in chemical complexity.
Nature’s solution for a more ecological, economical and innovative manufacturing is the integration of biocatalysis. This word describes a chemical transformation using an organic catalyst called a biocatalyst, proteins that are chemically active and thus able to replace chemical and inorganic catalysts.
Another key advantage of biocatalysts compared to inorganic catalysts is their inherent specificity, which makes them ideal for regioand stereoselective modifications. Thus, biocatalysis offers an attractive alternative to traditional chemistry, especially when precise and environmentally friendly reactions are desired.
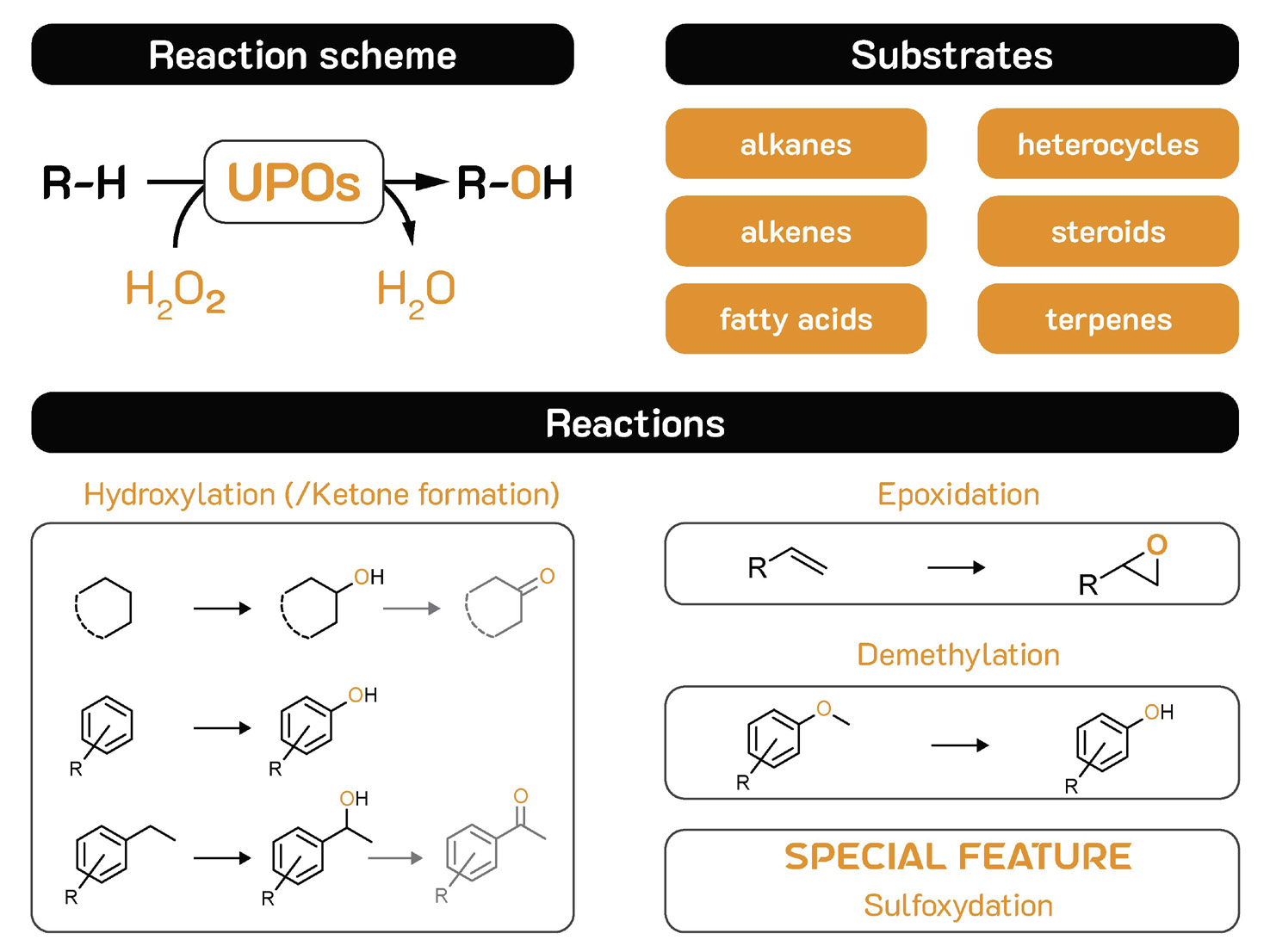
Biocatalytic oxyfunctionalisation
One particular area where biocatalysis has significant potential to outcompete chemical approaches is the functionalisation of compounds with oxygen. Chemical oxygenation typically requires hazardous conditions involving expensive catalysts. It often results in low product yields and a variety of bye-products, requiring substantial purification efforts.
In contrast, biocatalysts have the ability to oxygenate regio- and stereoselectively, particularly non-activated C-H bonds. This is relevant to pharmaceuticals, speciality chemicals, flavours, fragrances, antibiotics, mRNA vaccines, fatty acids, terpenes, nutraceuticals and sugars.
The ideal biocatalysts for hydroxylation are scalable, robust,
Figure 1 - Reaction scheme, substrates & reactions of UPOs .58 SPECIALITY CHEMICALS MAGAZINE ESTABLISHED 1981