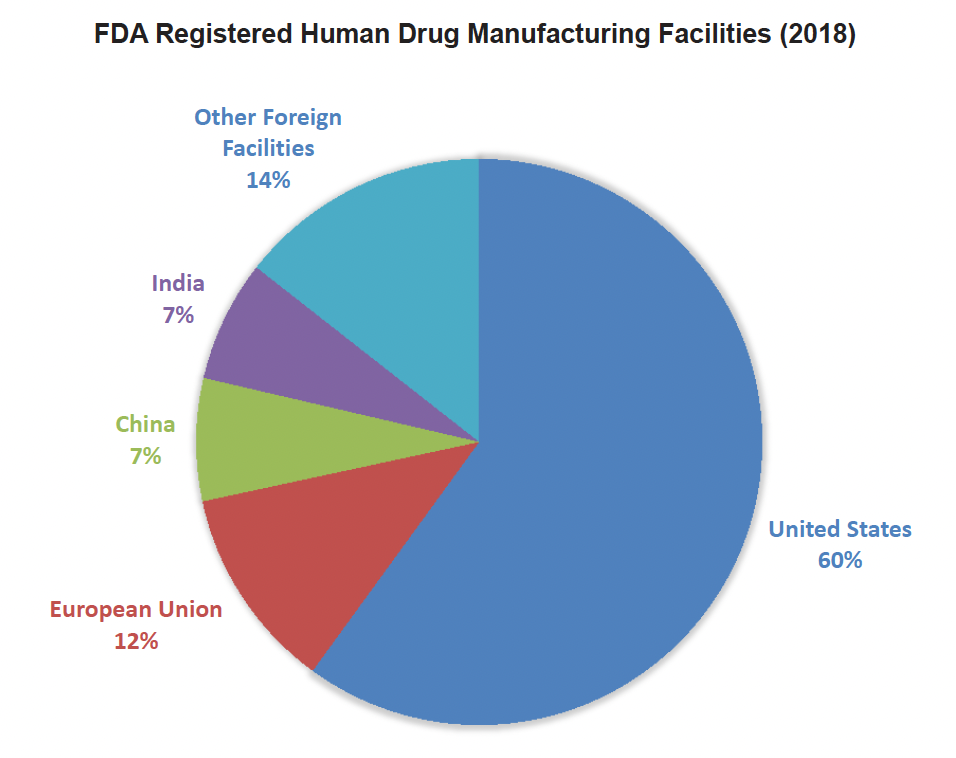
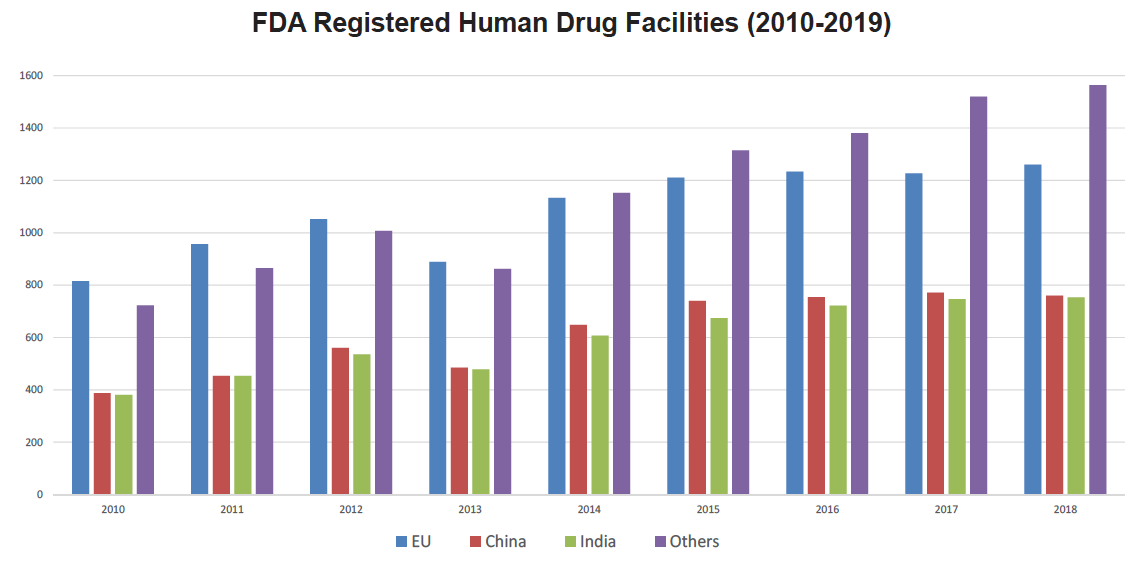
The advancing CMC innovation support programs at EMA and FDA are highly complementary in focus and structure, with distributed and continuous manufacturing drawing significant up-front attention at both agencies.
On both sides of the Atlantic, a significant effort is underway to deepen the regulator/industry/academia communication needed to realize the potential for technological advancement in the manufacturing and control arena. In this four-part story, IPQ explores the latest advancements in the EMA and FDA innovation support programs and the priorities that are now drawing front-burner attention.
PART I: European Regulatory Network Support for Manufacturing Innovation
The first part of the story highlights the evolution of regulatory support for biomanufacturing in Europe, sharing insights from two leading European biotech CMC reviewers. Included are the outcomes of the first year of operation of EMA’s Quality Innovation Group and its inaugural “Listen and Learn Focus Group” meeting on continuous and decentralized manufacturing, in which European, U.S. and Japanese regulators, industry, and academia participated. Pharma legislation has been proposed by the EC that targets further support for pharmaceutical innovation, including decentralized manufacturing.
PART II: FDA’s Expanding Engagement with Advanced Technologies
Part II provides an update on FDA’s efforts in the advanced manufacturing arena, with insights from key leaders in the Office of Pharmaceutical Quality (OPQ) and Office of Regulatory Science and Innovation (ORSI) that were shared at an October workshop on continuous manufacturing (CM) of nanomaterials. Reviewed is the attention CM has been receiving from the CDER Emerging Technologies Program since it was established in 2014 through its engagement with ICH Q13.
Also reviewed are:
- CDER’s July 2023 guidance on the use of voluntary consensus standards to advance pharmaceutical quality
- an ASTM standard on CM for biopharmaceuticals
- OPQ considerations for PAT in CM, and
- outcomes of a June 2023 Duke-Margolis/FDA workshop on manufacturing innovation.
PART III: Barriers to CM Adoption Explored at USP/RAPS Workshop
The third part of the story homes in on the discussions that took place at a USP/RAPS workshop in July, which brought together a variety of pharma stakeholders to explore barriers to continuous manufacturing (CM) adoption and identify pathways for addressing them. Emerging into clear relief during the course of the two-day workshop were the potential benefits that CM implementation could offer in terms of flexibility, efficiency, and quality, as well as the challenges, such as knowledge gaps, start-up costs, ROI, and global regulatory uncertainties.
Part IV: Distributed/POC Manufacturing and the CMC/Quality Regulatory Paradigm
The last part of the story takes a deeper look at how the new wave of biotherapeutics and personalized medicine is driving a paradigm shift toward decentralized/point-of care (POC) manufacturing and the adaptations that are needed in CMC/regulatory processes to accommodate a DM/POC approach.
Like continuous manufacturing, distributed/POC manufacturing has been drawing front-burner FDA attention in its efforts to help clear technology innovation pathways and realize product and process potentialities, and an “action plan” based on stakeholder feedback was released this fall. Providing a wealth of insights on the DM/POC issues are two former FDA CMC regulators who have been heavily engaged in the innovation regulatory dialogue, Richard McFarland, now with the Advanced Regenerative Manufacturing Institute (ARMI), and Christine Moore, now with Organon.